Spontaneous Cervical Artery Dissection:
A Fluoroquinolone Induced Connective Tissue Disorder?
SOURCE: Chiropractic & Manual Therapies 2018 (Jul 9); 26: 22
James S. Demetrious, DC, FACO
Wilmington, NC, USA.
BACKGROUND: Spontaneous cervical artery dissections more often manifest in young people and have been associated with catastrophic consequences. Some indeterminate risk factors have been identified, making the diagnosis of developing dissections quite difficult. Fluoroquinolone antibiotics have been recognized for their degradative effects on connective tissue. Recent studies have implicated fluoroquinolones in the genesis of aortic artery aneurysms. It is the purpose of this paper to provide reasoning for a testable hypothesis of whether fluoroquinolones constitute a risk factor associated with cervical artery dissections.
METHODS: A PubMed search was conducted to investigate whether cervical artery dissection has been associated with fluoroquinolone use. An assessment of risk factors was made of hereditary connective tissue disorders, infection, and seasonal predisposition related to cervical artery dissection. These factors were considered in conjunction with reports of connective tissue toxicity associated with fluoroquinolone medications.
RESULTS: It appears that no reported cases of cervical artery dissection have previously been correlated with fluoroquinolone use. Heritable connective tissue disorders, infection, seasonal predisposition and condition latencies are associated with fluoroquinolone medications. Several recent articles have implicated fluoroquinolones with aortic dissections and aneurysm.
There are more articles like this @ our:
CONCLUSION: A causal relationship of fluoroquinolone antibiotics to cervical artery dissection is plausible. The suppositions developed in this paper are insufficient to suggest that fluoroquinolones currently represent an established risk factor in the development of cervical artery dissections. Fluoroquinolones may indeed be a novel and previously unrecognized cause of cervical artery dissections.
KEYWORDS: Aneurysm; Carotid; Cervical artery dissection; Collagen; Connective tissue; Fluoroquinolone; Infarct; Stroke; Vertebral
From the FULL TEXT Article:
Introduction
The primary objective of this paper is to provide a testable hypothesis whether fluoroquinolone antibiotics constitute a risk factor associated with spontaneous cervical artery dissections.
Epidemiology
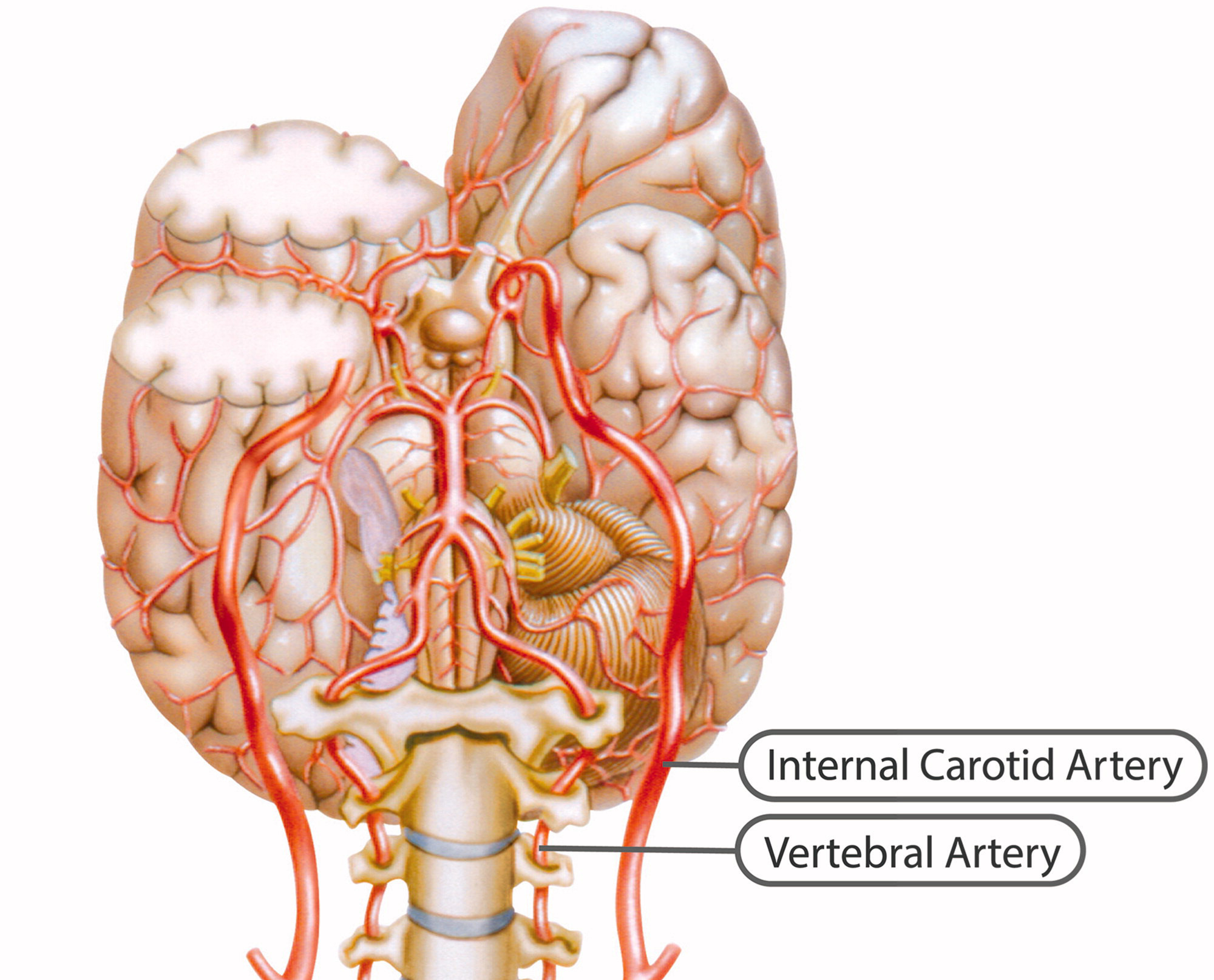
Spontaneous cervical artery dissections are a common cause of stroke in young adults, with a mean age of occurrence of approximately 45 years and a slight gender predisposition favoring males 48–55%. [1, 2] During a 17–year study period, Lee et al. reported a mean annual incident rate of 2.6 to 3.1 per 100,000 population. [3] A seasonal variation has been reported with cervical artery dissection more likely to occur in the winter. [4]
Congenital risk factors
Heritable disorders such as Ehlers-Danlos syndrome, Marfan syndrome, osteogenesis imperfecta type 1, autosomal dominant polycystic kidney disease, and fibromuscular dysplasia have been reported as risk factors for cervical artery dissection. [2] While only 1–5% of patients with these connective tissue disorders have been identified with spontaneous vertebral artery dissection, 20% of patients have a yet unnamed connective tissue disorder. [5]
Environmental risk factors
A history of recent infection has been associated as a risk factor for cervical artery dissection. [6, 7] Extrapolating from a national database of outpatient antibiotic prescriptions in the United States, Suda et al. reported an average of 24.5% more antibiotic prescriptions were dispensed in the winter months than in the summer, over a 5–year study period with a mean outpatient consumption of quinolone antibiotics of 13.66%. [8] These combined factors may partially explain the seasonal variation and increased risk of dissection during winter months.
Fluoroquinolone adverse effects
Often prescribed for urinary, respiratory, dermatologic and other infections, severe adverse effects of fluoroquinolones have been reported in the U.S., causing the Food and Drug Administration to communicate advisories related to safety and utilization:
-
October 2008 – Warning on tendon injuries with fluoroquinolone antibiotics.
-
August 2013 – Warning on peripheral neuropathy injuries with fluoroquinolone antibiotics.
-
May 12, 2016 – FDA approves safety labeling changes for fluoroquinolones.
-
July 26, 2016 – FDA Drug Safety Communication: FDA updates warnings for oral and injectable fluoroquinolone antibiotics due to disabling side effects.
Currently, the FDA has advised that fluoroquinolones should be reserved for use in patients who have no other treatment options for acute bacterial sinusitis, acute bacterial exacerbation of chronic bronchitis, and uncomplicated urinary tract infections because the risk of severe side effects outweighs the benefits in these patients. For some serious bacterial infections, the benefits of fluoroquinolones outweigh the risks, and it is appropriate for them to remain available as a therapeutic option. [9]
Read the rest of this Full Text article now!


Leave A Comment