Chiropractic Spinal Manipulative Therapy for Migraine: A Study Protocol of a Single-blinded Placebo-controlled Randomised Clinical Trial
SOURCE: BMJ Open. 2015 (Nov 19); 5 (11): e008095 ~ FULL TEXT
Aleksander Chaibi, Jurate Šaltyte Benth, Peter J Tuchin, Michael Bjørn Russell
Head and Neck Research Group,
Research Centre, Akershus University Hospital,
Lørenskog, Norway Institute of Clinical Medicine,
Akershus University Hospital,
University of Oslo,
Nordbyhagen, Norway
INTRODUCTION: Migraine affects 15% of the population, and has substantial health and socioeconomic costs. Pharmacological management is first-line treatment. However, acute and/or prophylactic medicine might not be tolerated due to side effects or contraindications. Thus, we aim to assess the efficacy of chiropractic spinal manipulative therapy (CSMT) for migraineurs in a single-blinded placebo-controlled randomised clinical trial (RCT).
METHOD AND ANALYSIS: According to the power calculations, 90 participants are needed in the RCT. Participants will be randomised into one of three groups: CSMT, placebo (sham manipulation) and control (usual non-manual management). The RCT consists of three stages: 1 month run-in, 3 months intervention and follow-up analyses at the end of the intervention and 3, 6 and 12 months. The primary end point is migraine frequency, while migraine duration, migraine intensity, headache index (frequency x duration x intensity) and medicine consumption are secondary end points. Primary analysis will assess a change in migraine frequency from baseline to the end of the intervention and follow-up, where the groups CSMT and placebo and CSMT and control will be compared. Owing to two group comparisons, p values below 0.025 will be considered statistically significant. For all secondary end points and analyses, a p value below 0.05 will be used. The results will be presented with the corresponding p values and 95% CIs.
There are more articles like this @ our:
ETHICS AND DISSEMINATION: The RCT will follow the clinical trial guidelines from the International Headache Society. The Norwegian Regional Committee for Medical Research Ethics and the Norwegian Social Science Data Services have approved the project. Procedure will be conducted according to the declaration of Helsinki. The results will be published at scientific meetings and in peer-reviewed journals.
TRIAL REGISTRATION NUMBER: NCT01741714
KEYWORDS: STATISTICS & RESEARCH METHODS
From the FULL TEXT Article:
Background
Migraine is a common health problem with substantial health and socioeconomic costs. On the recent Global Burden of Disease study, migraine was ranked as the third most common condition. [1]
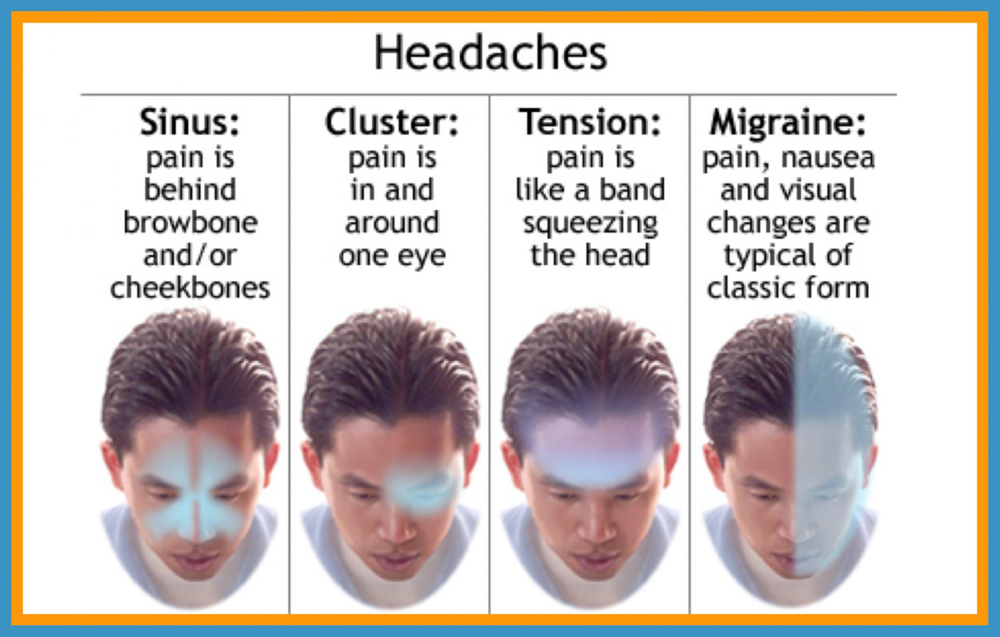
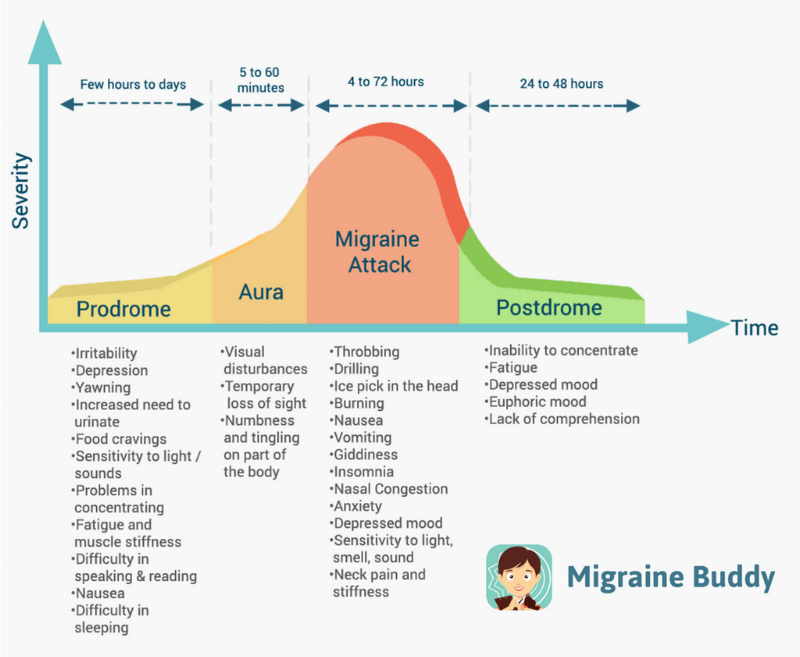
About 15% of the general population have migraine. [2 ,3] Migraine is usually unilateral with pulsating and moderate/severe headache which is aggravated by routine physical activity, and is accompanied by photophobia and phonophobia, nausea and sometimes vomiting. [4] Migraine exists in two major forms, migraine without aura and migraine with aura (Box 1). Aura is reversible neurological disturbances of the vision, sensory and/or speech function, occurring prior to the headache. However, intraindividual variations from attack to attack are common. [5, 6] The origin of migraine is debated. The painful impulses may originate from the trigeminal nerve, central and/or peripheral mechanisms. [ 7, 8] Extracranial pain sensitive structures include the skin, muscles, arteries, periosteum and joints. The skin is sensitive to all usual forms of pain stimuli, while temporal and neck muscles may especially be sources for pain and tenderness in migraine. [9–11] Similarly, the frontal supraorbital, superficial temporal, posterior and occipital arteries are sensitive to pain. [9, 12]
Read the rest of this Full Text article now!

Leave A Comment