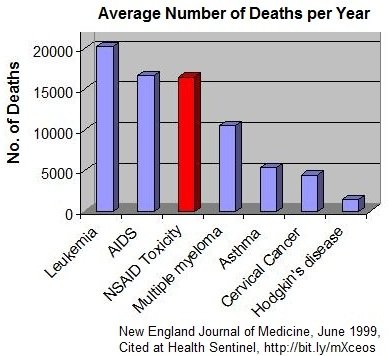
Quantifying the Impact of NSAID-associated Adverse Events
Quantifying the Impact of NSAID-associated Adverse Events
SOURCE: Am J Manag Care. 2013 (Nov); 19 (14 Suppl): s267–272
Michael Fine, MD
Health Net
736 Kendall Dr
Laguna Beach, CA 92651
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used among patients experiencing many different types of pain, including inflammatory, acute pain (eg, injury, low back pain, headache, postoperative pain), and chronic pain (eg, rheumatoid arthritis, osteoarthritis).
However, both traditional NSAIDs and second-generation NSAIDs (cyclooxygenase-2 inhibitors) can lead to very expensive and serious adverse events. Gastrointestinal, cardiovascular, and renal complications associated with NSAIDs have been shown to be dose-dependent. In 2005, to help minimize these risks, the US Food and Drug Administration issued a public health advisory stating that “NSAIDs should be administered at the lowest effective dose for the shortest duration consistent with individual patient treatment goals.”
This article reviews the undue clinical and economic burden associated with NSAID-related serious adverse events.
From the FULL TEXT Article:
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the cornerstone of pain management in patients who have inflammatory, acute pain (eg, headache, postoperative pain, and orthopedic fractures), and chronic pain (eg, rheumatoid arthritis, osteoarthritis, and gout). [1, 2] Approximately 70% of people 65 years or older use NSAIDs at least once per week, with half of them taking at least 7 doses per week. In 2000, more than 111 million prescriptions were written for NSAIDs in the United States, at an approximate cost of $4.8 billion. [3] The use of NSAIDs is likely to increase even more as the US population continues to age and experience painful conditions that are more common among older adults. [4]
There are more articles like this @ our: